
Michael Jordan – Age, Bio, Birthday, Family, Net Worth
Michael Jordan, the legendary basketball player, needs no introduction. His name is synonymous with greatness in the world of sports. ...
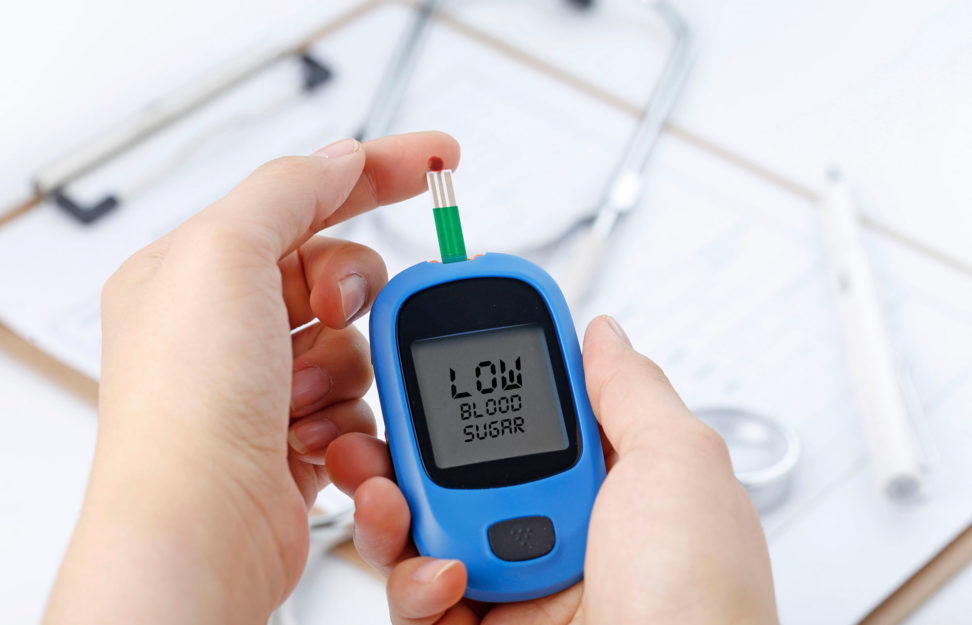
Type 2 Diabetes: Causes, Symptoms And Treatment
Type 2 diabetes is a chronic medical condition that affects millions of people worldwide. It is a metabolic disorder characterized ...

Quando Rondo – Age, Bio, Birthday, Family, Net Worth
Quando Rondo is a rising star in the world of hip-hop, known for his unique style and captivating lyrics. Born ...

How to Make a Walmart-to-Walmart Money Transfer
In today’s fast-paced world, the need for convenient and secure money transfer services is more significant than ever. Whether you ...

Alison Victoria Biography: Age, Birthday, Early Life, Career Opportunities, Source of Income, Net Worth
Alison Victoria, a name synonymous with interior design and home renovation, has captured the hearts of millions with her charisma ...

Dr Stone Season 4 Release Date,Cast, Plot, Trailer, Episodes 2023
Dr. Stone, the hit anime series that has captured the hearts of fans around the world, is gearing up for ...

Why is Netflix Removing Christian Movies? Check out Facts vs Rumors
In recent times, rumours have circulated that Netflix is systematically removing Christian movies from its streaming platform. These rumours have ...

Jacob Elordi – Age, Bio, Birthday, Family, Net Worth
Jacob Elordi has quickly risen to stardom in Hollywood, captivating audiences with his impressive acting skills and striking looks. Born ...

National Daughters Day — Why, How and When to Celebrate
National Daughters Day is a special occasion that celebrates the daughters in our lives and recognizes their importance in our ...

George Michael – Age, Bio, Birthday, Family, Net Worth
George Michael, a name synonymous with timeless music and an iconic voice, remains a beloved figure in the music industry. ...

George Strait – Age, Bio, Birthday, Family, Net Worth
George Strait, often referred to as the “King of Country,” is an iconic figure in the world of country music. ...

5 Ways Patriotic T-Shirts Can Empower Women- Expressing Identity
Are you a woman who wants to proudly showcase your patriotism while embracing your individuality? Look no further! In this ...

John Denver – Age, Bio, Birthday, Family, Net Worth
John Denver, a name synonymous with the gentle melodies of folk and country music, left an indelible mark on the ...

Hong Chau Biography: Age, Husband, Parents, Net Worth
In the world of Hollywood, where talent shines bright, Hong Chau is a name that has steadily risen to prominence. ...

Jason Momoa – Age, Bio, Birthday, Family, Net Worth
Jason Momoa is a name that resonates with strength, charisma, and a magnetic presence in Hollywood. Known for his roles ...

How To Get Urgent Loans With Bad Credit in 2023?
Financial emergencies can strike at any time, leaving you in a tight spot. Whether it’s unexpected medical bills, car repairs, ...

Vincent van Gogh – Age, Bio, Birthday, Family, Net Worth
Vincent van Gogh, a name synonymous with artistic brilliance, is a figure who has left an indelible mark on the ...

International Chocolate Day 2023: Date, History, Facts, Activities
Chocolate lovers around the world unite! International Chocolate Day, celebrated on a global scale every year, is an occasion that ...

Blippi – Age, Bio, Birthday, Family, Net Worth
Blippi, the beloved children’s entertainer, has taken the world by storm with his educational and entertaining videos that captivate young ...

Charles Halford Biography: Age, Height, Career, Family, Personal Life, Net Worth
In the world of entertainment, there are a few individuals who leave a lasting impact not just through their talent ...

Chuck Todd Biography: Age, Birthday, Personal Life, Career, and Achievements
In the fast-paced world of journalism, few names stand out as prominently as Chuck Todd. With a career spanning decades, ...

Ryan Reynolds Biography: Age, Height, Weight, Wife, Girlfriend, Family, Net worth
Ryan Reynolds, the charismatic Canadian actor, is a household name in Hollywood and the film industry worldwide. With his infectious ...

Tracee Ellis Ross – Age, Bio, Birthday, Family, Net Worth
In the world of Hollywood, few names shine as brightly as Tracee Ellis Ross. Known for her exceptional talent, elegance, ...

Canelo Álvarez – Age, Bio, Birthday, Family, Net Worth
Canelo Álvarez, whose full name is Santos Saúl Álvarez Barragán, is a Mexican professional boxer who has taken the boxing ...

Scarlett Johansson – Age, Bio, Birthday, Family, Net Worth
Scarlett Johansson is a household name in Hollywood, known for her incredible talent, stunning beauty, and versatile acting skills. In ...

21 Savage – Age, Bio, Birthday, Family, Net Worth
Step into the intriguing world of one of rap’s most enigmatic stars as we unravel the enigma that is 21 ...

Karl Lagerfeld Biography: Age, Height, Birthday, Family, Net Worth
Karl Lagerfeld, an iconic figure in the world of fashion, left an indelible mark on the industry with his creative ...

Corey Feldman – Age, Bio, Birthday, Family, Net Worth
Corey Feldman is a household name in the entertainment industry, known for his charismatic on-screen presence and versatile talent. With ...

Little Richard – Age, Bio, Birthday, Family, Net Worth
Little Richard, the flamboyant pioneer of rock and roll, left an indelible mark on the music industry. Born as Richard ...

Your Ultimate Guide to Wrigley Field Concert Seating Chart
Wrigley Field, the iconic home of the Chicago Cubs, is not just reserved for baseball games. It’s also a epic ...
-
Michael Jordan – Age, Bio, Birthday, Family, Net Worth

Michael Jordan, the legendary basketball player, needs no introduction. His name is synonymous with greatness in the world of sports. In this article, we will delve into the life and achievements of this iconic figure, exploring his age, bio, birthday, family, and net worth. Michael Jordan’s Early Life Born on February 17, 1963, in Brooklyn,…
-
Type 2 Diabetes: Causes, Symptoms And Treatment

Type 2 diabetes is a chronic medical condition that affects millions of people worldwide. It is a metabolic disorder characterized by high levels of blood sugar (glucose) resulting from the body’s inability to effectively use insulin. In this comprehensive article, we will explore the causes, symptoms, and various treatment options available for managing type 2…
-
Quando Rondo – Age, Bio, Birthday, Family, Net Worth

Quando Rondo is a rising star in the world of hip-hop, known for his unique style and captivating lyrics. Born Tyquian Terrel Bowman, he has taken the music industry by storm with his impressive talent and compelling life story. In this article, we will delve into Quando Rondo’s age, bio, birthday, family, and net worth,…







